MK Research Group
Electrocatalysis Lab
Mechanistic analysis of the ORR
Oxygen reduction reaction (ORR) is one of the most studied reactions in electrocatalysis. The most abundant element, oxygen, is used as the oxidizing agent in the low-temperature hydrogen fuel cells. Typically, platinum-based catalysts have been used to improve the ORR kinetics, limiting large-scale commercialization of fuel cells for energy applications. Understanding the ORR mechanism and the information about the active sites on NPGM or metal-free catalysts represents a significant bottleneck in developing highly active and durable Pt-free catalysts for the ORR. Despite the series of attempts to characterize the active sites and ORR mechanism, the conclusions are not clear and lead to debate.
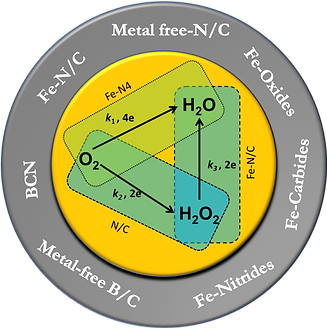
Dr. Muthukrishnan’s group is working on fundamental aspects of ORR, characterizing the active sites and mechanism of the Fe-N/C and N-doped carbon catalysts via kinetic analysis. A bottom-up approach to describe the role of various possible entities present in the heat-treated heteroatom-doped Fe-containing catalysts is individually studied.
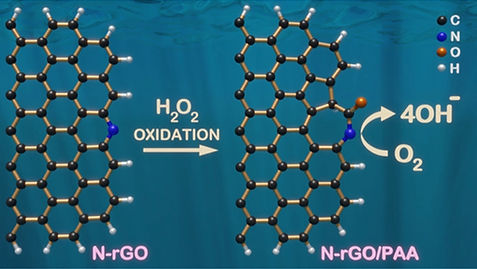
Also, the mechanism of the synergistic effect on the two heteroatom-doped (boron and nitrogen) metal-free carbon catalysts. To specific, the BCN materials are studied, and its kinetic analysis reveals the mechanism of the synergistic effect.The defects on the carbon substrates towards ORR activity are analyzing by specially created defects. The selective edge functionalization of heteroatom-doped graphene was employed to create the topological defects, which significantly improves the ORR activity in alkaline medium. (highlighted in figure)
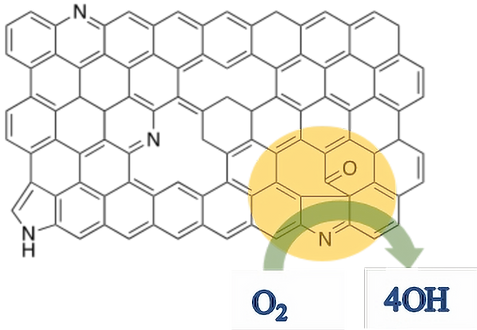
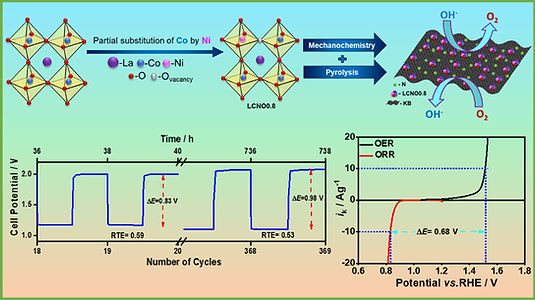
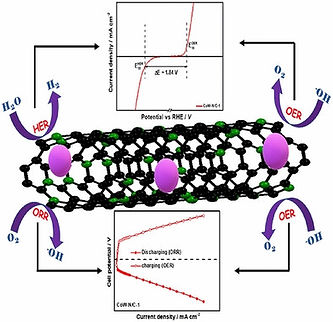
Development of trifunctional Catalysts
The effective operation of energy conversion and storage devices like the Zn-air battery and water electrolyzer relies on three principal electrochemical redox reactions: the oxygen reduction reaction (ORR), the oxygen evolution reaction (OER), and the hydrogen evolution reaction (HER). In particular, the ORR and OER take place in metal-air batteries, the ORR occurs in the cathode of fuel cells, and water splitting involves the OER and HER.
Materials capable of efficiently catalyzing ORR, OER and HER reactions hold great promise in the realm of new energy technologies like self-driven water electrolysis and integrated battery/electrolyzer systems. Many materials have been popularized as trifunctional catalysts, such as oxides, sulfides, phosphides, nitrides, layered double hydroxides (LDHs), metal–organic frameworks (MOFs) and single atoms. The group primarily focuses on transition metal-based MOFs as well as Rare-earth and transition metal-based oxides.
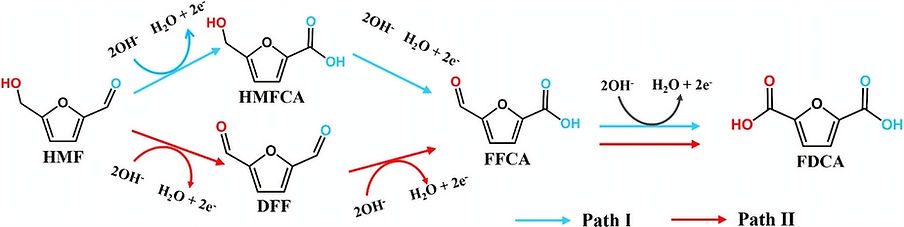
Biomass Oxidation and its mechanistic analysis
In our lab, we investigate the electrochemical valorization of biomass-derived 5-hydroxymethylfurfural (HMF) into 2,5-furandicarboxylic acid (FDCA), a key monomer for bio-based plastics. A central focus of our work is unraveling the mechanistic landscape of this transformation by distinguishing between the competing oxidation pathways—via HMFCA/FFCA (Path I) and DFF (Path II). By integrating electrochemical techniques with in situ/operando spectroscopy, we aim to selectively steer the reaction toward the most efficient and sustainable route for FDCA production.